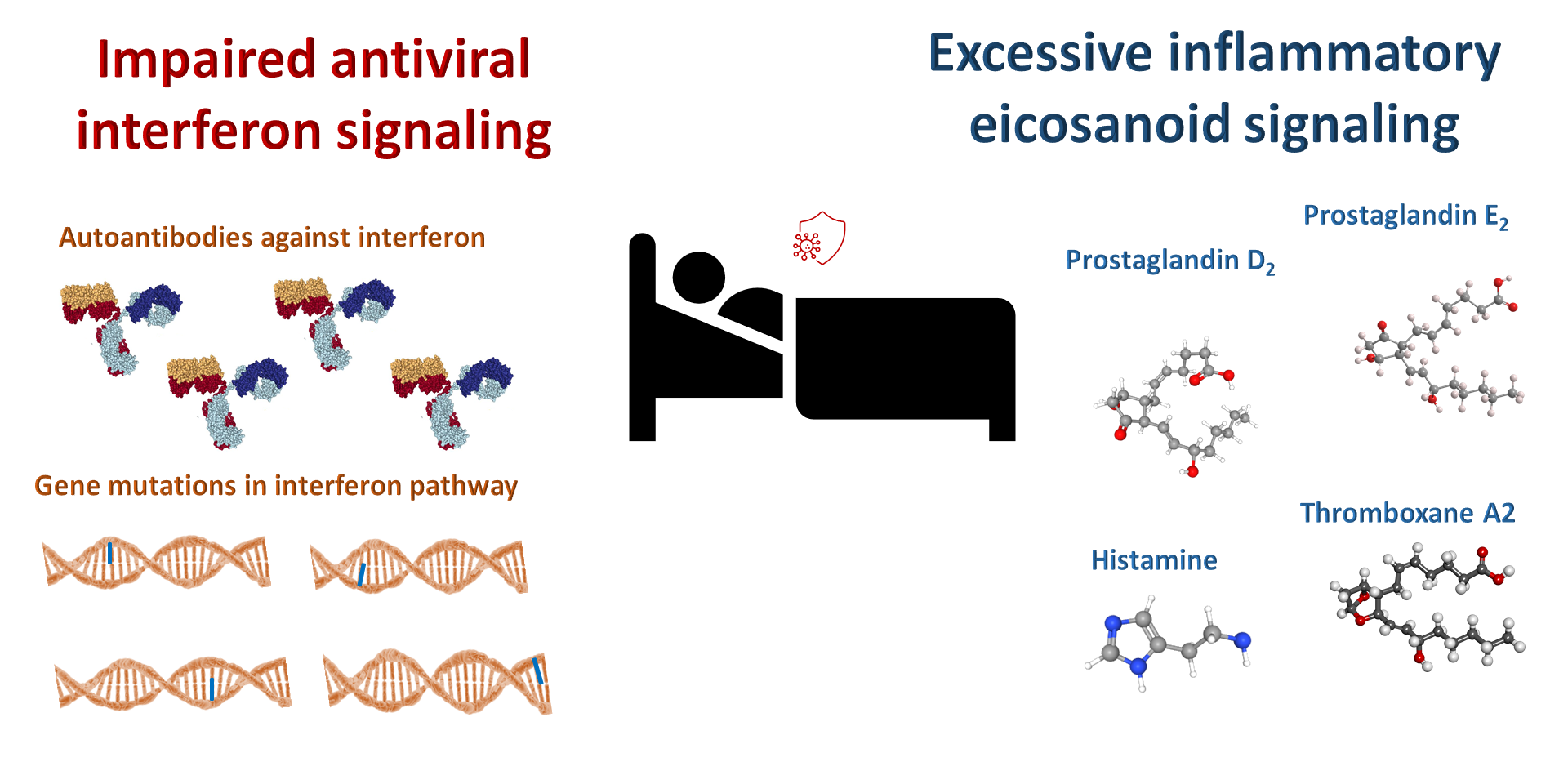
Too much histamine or prostaglandin D2 signaling or too little interferon signaling lead to increased risk for severe COVID-19.
An imbalanced immune response appears to be the key to whether a person develops severe COVID-19 with pneumonia and other complications such as coagulopathy (excessive formation of blood clots), impaired kidney function, neurological issues, and heart complications. Multiple mechanisms likely contribute to the variability in the immune response within the population and determine whether a person has asymptomatic disease, mild disease, or severe disease with potentially fatal complications. Increased understanding of the immune response to infection by the SARS-CoV-2 response will enable clinicians to provide optimal treatment to rebalance the response, either preventing people infected with the virus from developing a serious case of COVID-19 or providing appropriate effective treatment if someone does.
Here, I focus on three of these: An impaired interferon response, an excessive mast cell response, and excess signaling through a specific prostaglandin receptor.
Ineffective interferon response
An ineffective early interferon type I response is one path that leads to severe COVID-19. Interferons are a family of proteins that provide the first response to control viral infections, engaging immune cells to kill the virus and inducing pathways that enable the immune system to identify and eliminate infected cells to control the spread of the virus.
Genetic studies identified mutations in genes in this interferon pathway that impair this critical antiviral response, leading to severe COVID-19. Eventually a genetic test that includes the mutations so far identified (and likely others yet to be identified) may become available to screen for COVID-19 susceptibility and design the optimal treatment and prioritize vaccination of those people at increased risk.
Others discovered autoantibodies against type I interferons that neutralize the function of these antiviral proteins. The autoantibodies can be easily identified in a blood sample and provide key information for effectively treating such people who develop COVID-19 and prevent patients that recover from COVID-19 who have these autoantibodies from donating plasma for treating other patients.
Hyperactive mast cell response
Another path that may lead to an inappropriate immune response is aberrant hyperactivation of mast cells. Mast cells are immune cells that respond to many of the inflammatory mediators associated with the hyperinflammatory state called “cytokine storm,” which is a major contributor to COVID-19 mortality.
In addition to responding to inflammatory signals, mast cells produce and release inflammatory signals. Thus, mast cells also contribute to inflammation by releasing pro-inflammatory mediators that signal to other immune cells and affect non-immune cells, such as the endothelial cells that line the blood vessels.
One that is particularly relevant in the lungs of COVID-19 patients is the effect of molecule released by mast cells on the endothelial cells of the blood vessels. Mast cell signals increase the permeability of blood vessels, which helps immune cells get into tissues where infection is happening. Although getting immune cells into sites of infection is how increased blood vessel permeability is beneficial; in the lungs of COVID-19 patients, increased blood vessel permeability can contribute to difficulty breathing and damage to the alveoli, the structures in the lungs where gases are exchanged (oxygen in and carbon dioxide out).
Indeed, multiple aspects of severe COVID-19 resemble a disorder called “mast cell activation syndrome” (MCAS). One theory about COVID-19 “long-haulers” is that these patients develop a form of post-infection MCAS that flares causing symptoms after the infection has resolved.
One of the key inflammatory mediators released by mast cells is histamine. One of the experimental therapies involves famotidine, a drug that binds to a specific class of histamine receptors, the H2 receptors. Famotidine inhibits one path of H2 signaling and partially stimulates another path of H2 signaling. Inhibiting pro-inflammatory signaling by histamine released from hyperactivated mast cells is the basis for testing famotidine as a treatment for early and late stages of COVID-19 and preventing escalation of symptoms related to excessive inflammation. This drug is currently in clinical trials for COVID-19 (NCT: https://clinicaltrials.gov/ct2/show/NCT04370262), and President Trump received it as part of his treatment.
Altered eicosanoid response
Eicosanoids are a class of structurally related bioactive lipids. These molecules are synthesized from a lipid called arachidonic acid that is released from cellular membranes. Among other molecules, eicosanoids include prostaglandins, thromboxanes, and leukotrienes.
Prostaglandins are part of a large structurally related group of bioactive lipid molecules. In addition to the diversity of individual prostaglandins, these molecules interact with multiple receptors on their target cells. Thus, the effect of prostaglandins depends not only on which enzymes are present to make a specific prostaglandin molecule but which receptors are present on the cell that responds to the prostaglandin. Thus, individual prostaglandins can have both pro-inflammatory and anti-inflammatory effects, depending on which receptors are present and which cells are responding.
This duality in prostaglandin function is why many people have stomach pain when they take medicines that inhibit cyclo-oxygenase (like aspirin, ibuprofen, and naprosyn). These drugs block production of all prostaglandins, because cyclo-oxygenases are the enzymes that act at the beginning of the pathway for all of these bioactive lipids. Along with blocking the production of prostaglandins that cause pain, fever, swelling, and inflammation, these medications also block the prostaglandins that help protect the stomach.
Mast cells respond to and release prostaglandins. Prostaglandin E2 (PGE2) stimulates mast cells to release their inflammatory mediators, which include histamine and prostaglandin D2. Some patients treated with famotidine also received an inhibitor of one of the two enzymes involved in production of eicosanoid bioactive lipids. They received celecoxib (brand name Celebrex), which inhibits cyclo-oxygenase 2 (COX-2). Celecoxib also inhibits another enzyme that is responsible for production of leukotrienes.
The goal of using celecoxib rather than aspirin or another cyclo-oxygenase inhibitor is that celecoxib inhibits only one of the two cyclo-oxygenase enzymes. Importantly, celecoxib inhibits the enzyme COX-2, which is the form that increases in cells infected with SARS-CoV-2, the virus that causes COVID-19.
One benefit of inhibition of COX-2 is reducing the production of PGE2, which reduces activation of mast cells. Another is reducing the production of thromboxanes that trigger platelet aggregation and blood clotting.
Not all patients who develop severe COVID-19 may have hyperactive mast cells. Other imbalances in inflammatory signaling could be involved. For example, changes in the production of prostaglandins are associated with aging and obesity. A prostaglandin that shows an increase in aging and obesity is called PGD2. Thus, increased PGD2 could be the reason why older people and overweight people are at high risk for developing severe COVID-19 and COVID-19 mortality.
PGD2 is synthesized by many different cells. In the immune system, many cells produce PDG2. In the innate part of the immune system, PGD2-producing cells include mast cells and other cells involved in inflammatory responses. In the adaptive part of the immune system, a type of cell called T helper type 2 cells produces PGD2. Cells outside of the immune system also produce PGD2. For example, PDG2 is also produced by epithelial cells, such as those that line the respiratory tract.
The role of PGD2 in the response to infection is complicated, because PGD2 can bind to three different receptors with different functions. One of the receptors, DP1, inhibits platelet aggregation (reduces blot clotting), inhibits histamine release from mast cells, and relaxes smooth muscle, which would make breathing easier. These are all beneficial effects in someone with COVID-19. However, the other two receptors, DP2 and TP, have functions that are detrimental in someone with COVID-19. Thus, blocking the effects of PGD2 mediated by DP2 and TP could be beneficial in COVID-19; whereas blocking the effect through DP1 would be detrimental.
Through the receptor called DP2, PGD2 suppresses immune responses that would help eliminate the virus that causes COVID-19. A study in mice and human infants showed that PGD2 signaling through DP2 contributed to the severity of respiratory viral infection by inhibiting the production of a specific interferon (IFN-λ) from infected epithelial cells in the airway.
In the lung, high amounts of PGD2 promote bronchoconstriction through TP. It is unlikely that PGD2 concentrations are high enough in the blood to trigger excess blood clotting. However, TP is also the receptor for another bioactive lipid called thromboxane A2. Activation of TP in platelets by thromboxane A2 is critical for blood clotting. However, in COVID-19 patients excessive blood clotting occurs. Thus, the anticoagulating (blood thinning) effect of blocking this receptor would be beneficial.
Because PGD2 has been implicated in various types of allergic responses, including nasal allergies and asthma, a drug that blocks two of its receptors, TP and DP2, is approved for use in Japan to treat nasal allergy symptoms. This drug is called ramatroban (brand name Baynas) and has been used for more than 20 years in Japan.
Gupta and colleagues argue that clinical trials should test ramatroban for preventing disease progression and treating COVID-19. Because inhibition of both TP and DP2 would be beneficial in preventing respiratory symptoms, enhancing viral clearance, and limiting COVID-19-associated pathological blood clotting, ramatroban is well positioned to be effective. Because it has a long history of safe use in a broad spectrum of the population in Japan, it could rapidly be repurposed for use in COVID-19.
Indeed, ramatroban could be a better option than celecoxib or aspirin. Celecoxib and other non-steroidal anti-inflammatory drugs (NSAIDs) block prostaglandin synthesis at an earlier step. Thus, any beneficial effects of prostaglandins would be lost, along with the ones that contribute to COVID-19 severity.
Conclusion
With the increased understanding of the immune response to COVID-19, options for limiting the severity of COVID-19 and providing treatments that bridge the gap until safe and effective vaccines are widely available. Supplemental interferons, immune-suppressing steroids (such as dexamethasone), histamine receptor inhibitors such as famotidine, eicosanoid inhibitors like celecoxib, and specific prostaglandin receptor inhibitors like ramatroban may all provide treatment options that reduce the mortality and the fear associated with COVID-19.

References
Interferon
Bastard P, Rosen LB, Zhang Q … HGID Lab; NIAID-USUHS Immune Response to COVID Group; COVID Clinicians; COVID-STORM Clinicians; Imagine COVID Group; French COVID Cohort Study Group; Milieu Intérieur Consortium; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort … Casanova JL. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science (24 September 2020) DOI: 10.1126/science.abd4585
Zhang Q, Bastard P, Liu Z … COVID-STORM Clinicians; COVID Clinicians; Imagine COVID Group; French COVID Cohort Study Group; CoV-Contact Cohort; Amsterdam UMC Covid-19; Biobank; COVID Human Genetic Effort; NIAID-USUHS; TAGC COVID Immunity Group …Casanova JL. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science (24 September 2020) DOI: 10.1126/science.abd4570
Zhang Z, Ohto U, Shibata T, et al. Structural analysis reveals that Toll-like receptor 7 is a dual receptor for guanosine and single-stranded RNA. Immunity 45, 737–748 (2016). DOI: 10.1016/j.immuni.2016.09.011
Park and Iwasaki, Type I and Type III Interferons — Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host & Microbe (2020). DOI: 10.1016/j.chom.2020.05.008
Mast cells
Sethia R, Prasad M, Mahapatra SJ, et al. Efficacy of famotidine for COVID-19: A systematic review and meta-analysis. medRxiv (30 September 2020) DOI: 10.1101/2020.09.28.20203463 [Preprint]
Freedberg DE, Conigliaro J, Wang TC, … Famotidine Research Group. Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: A propensity score matched retrospective cohort study. Gastroenterology 159: 1129–1131.e3 (2020). DOI: 10.1053/j.gastro.2020.05.053
Mather JF, Seip RL, McKay RG. Impact of famotidine use on clinical outcomes of hospitalized Patients With COVID-19. Am. J. Gastroenterol. 115: 1617–1623 (2020). DOI: 10.14309/ajg.0000000000000832
Malone RW, Tisdall P, Fremont-Smith P, et al. COVID-19: Famotidine, histamine, mast cells, and mechanisms. Research Square (31 August 2020) DOI: 10.21203/rs.3.rs-30934/v3 [Preprint]
Tomera KM, Malone RW, Kittah JK. Brief Report: Rapid clinical recovery from severe COVID-19 with high dose famotidine and high dose celecoxib adjuvant therapy. Enliven: Pharmacovigilance and Drug Safety 6: 020 (2020). [Available as a preprint from Preprints 2020, 2020080519 (doi: 10.20944/preprints202008.0519.v1)]
Tomera KM, Malone RW, Kittah JK. Hospitalized COVID-19 patients treated with celecoxib and high dose famotidine adjuvant therapy show significant clinical responses. SSRN (version 1: 8 July 2020; version accessed 1 October 2020) DOI: 10.2139/ssrn.3646583
Morimoto K, Shirata N, Taketomi Y, et al. Prostaglandin E2-EP3 signaling induces inflammatory swelling by mast cell activation. J. Immunol. 192: 1130–1137 (2014). DOI: 10.4049/jimmunol.1300290
Prostaglandin D2
Hernandez-Carretero A, Weber N, La Frano MR, et al. Obesity-induced changes in lipid mediators persist after weight loss. International Journal of Obesity 42: 728–736 (2018). DOI: 10.1038/ijo.2017.266.
Zhao J, Zhao J, Legge K, Perlman S. Age-related increases in PGD2 expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J. Clin. Invest. 121:4921–4930 (2011). DOI: 10.1172/JCI59777. https://doi.org/10.1172/JCI59777
Gupta A, Kalantar-Zadeh K, Reddy ST. Ramatroban as a novel immunotherapy for COVID-19. J. Mol. Genet. Med. 14: 10.37421/jmgm.2020.14.457 (2020). DOI:10.37421/jmgm.2020.14.457
Werder RB, Lynch JP, Simpson JC, et al. PGD2/DP2 receptor activation promotes severe viral bronchiolitis by suppressing IFN-λ production. Sci. Transl. Med. 10: eaao0052 (2018). DOI: 10.1126/scitranslmed.aao0052.

